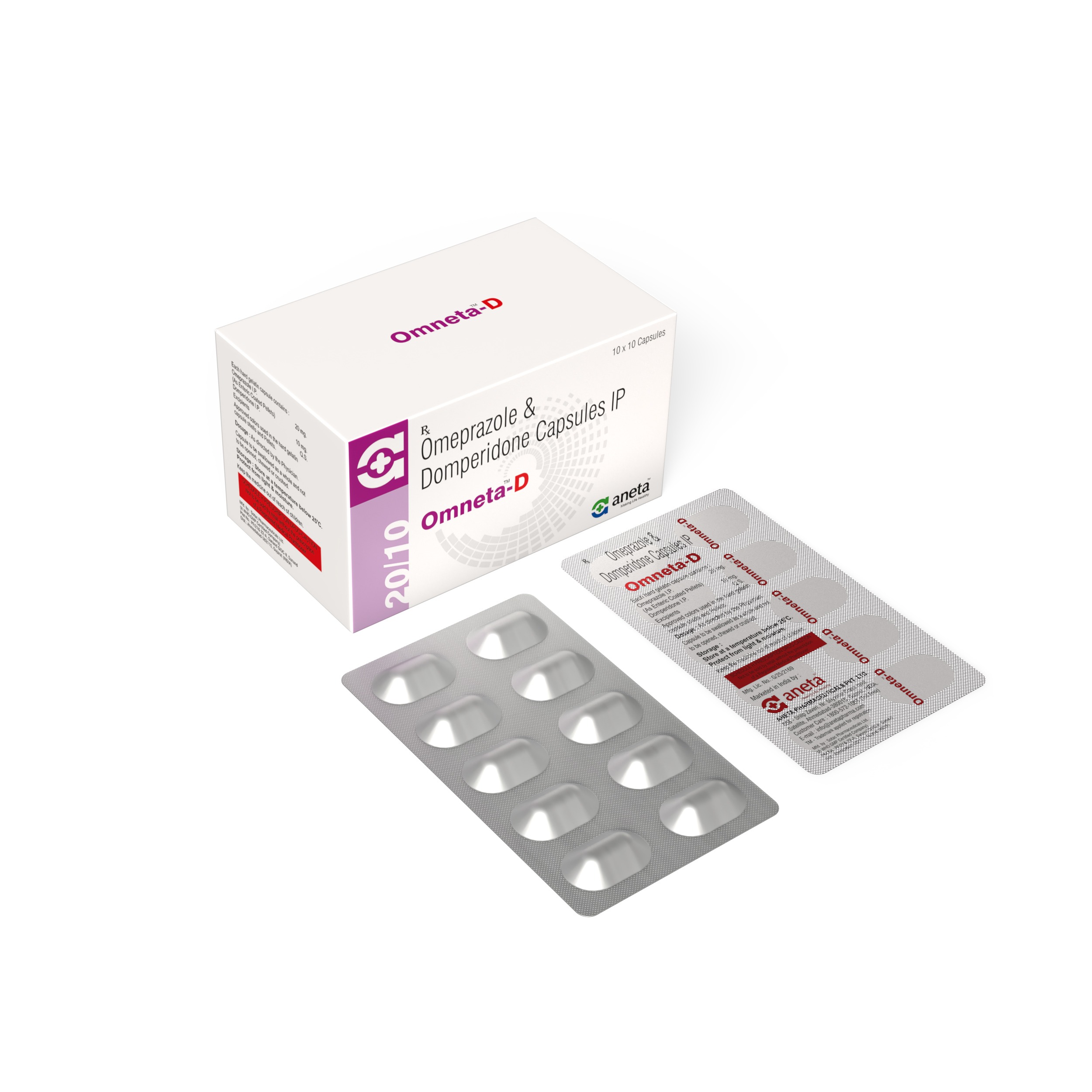
OMNETA
Omeprazole 20 mg - 10 Cap

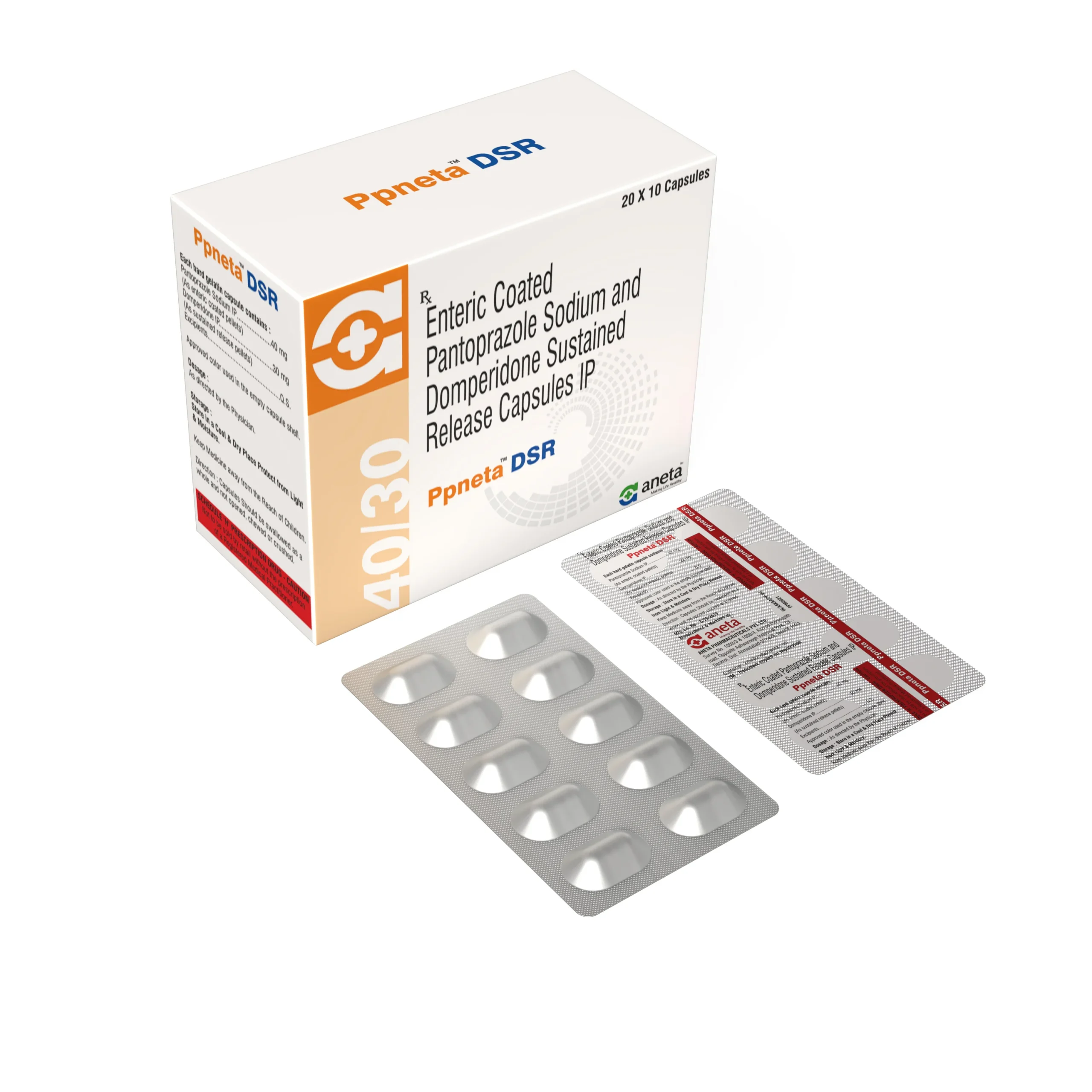
PPNETA LSR
Pantoprazole 40 mg + Levosulpiride 75 mg SR - 10 Cap

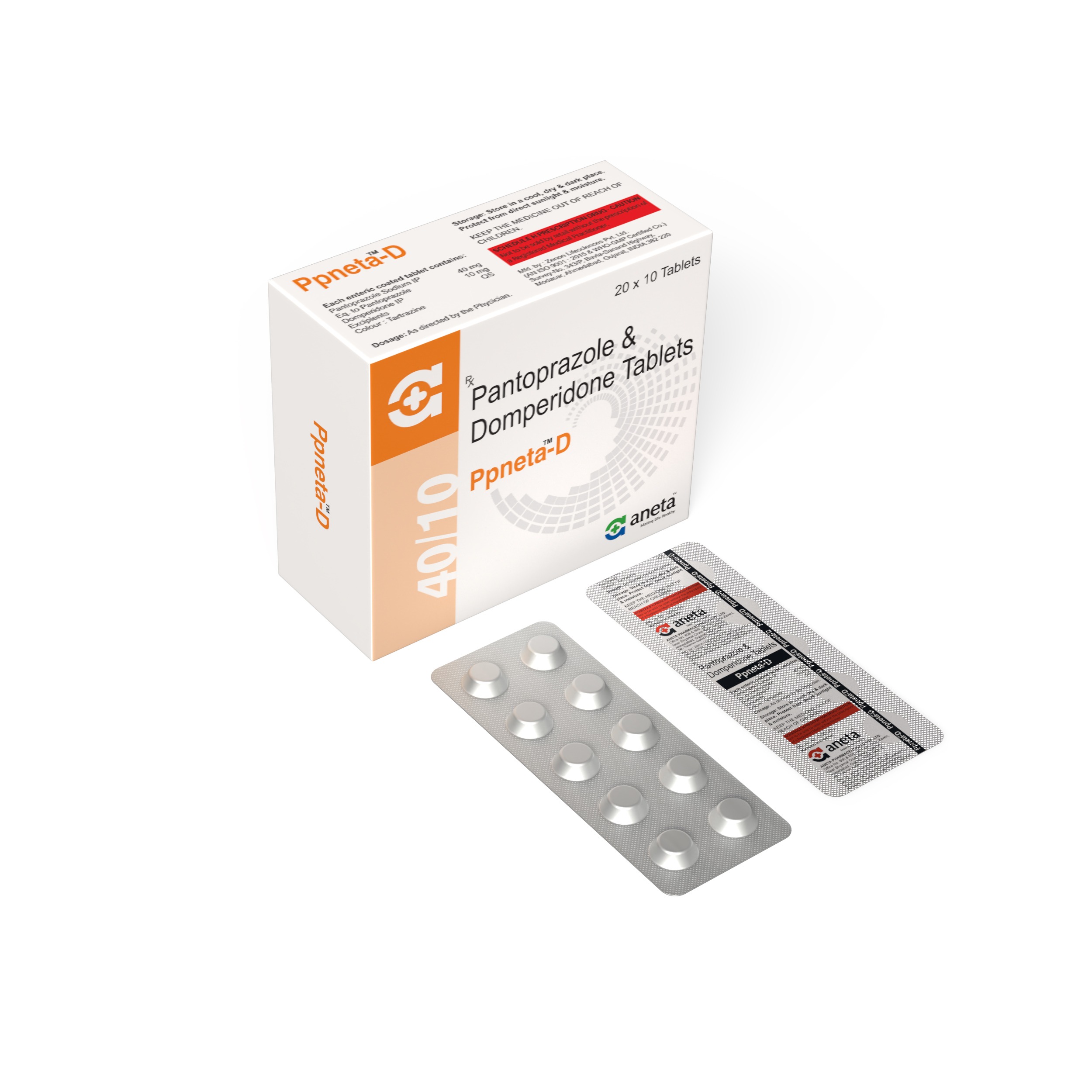
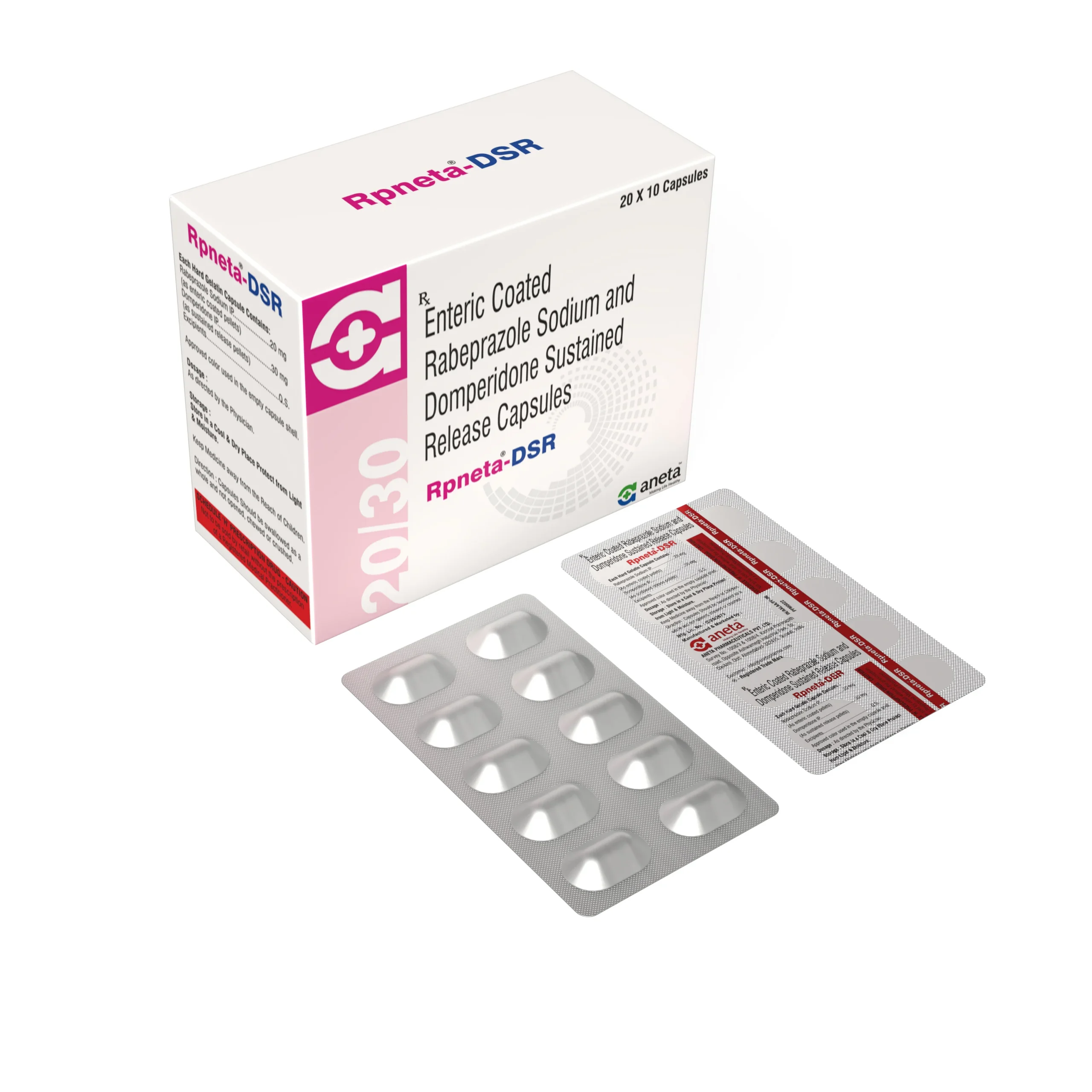
RPNETA D
Rabeprazole 20 mg + Domperidone 10 mg - 10 Tab
Aneta has in place a thorough quality control system and labs that cover every step of the production process, from raw materials to finished products. This involves testing and analyzing raw ingredients regularly and in-process and final product testing to verify that our products meet or exceed regulatory criteria for safety and efficacy.
Raw ingredients and finished products are tested using proven methods and equipment following defined procedures. Physical, Chemical, and Microbiological Tests are performed to confirm that the items fulfill the essential specifications for Identification, Purity, Potency, and Safety.
With the use of dedicated Equipment, Cleaning Methods, and Employee Training, our manufacturing facilities are intended to reduce the danger of cross-contamination. We also check the environment regularly to verify that our facilities satisfy set cleanliness requirements.
We have a comprehensive change control procedure in place that necessitates a full assessment of any proposed modifications to our production processes or equipment. This comprises assessing the possible impact on product quality, followed by validation and testing to verify that the modifications do not have a negative impact on product quality or safety.
For its raw ingredients, excipients, and immediate, Aneta has a stringent vendor qualification and monitoring procedure in place. This includes inspecting raw materials in its well-equipped QC lab as well as analyzing documentation and records of quality control methods supplied by the supplier. Aneta also has a system in place to continuously monitor and assess our suppliers to ensure that they continue to meet our quality requirements. Any non-compliance with raw material quality by suppliers is unacceptable to Aneta.
We have a strong quality management system in place to guarantee that all applicable regulatory standards are met. This involves keeping the necessary licenses and certifications, creating and adhering to standard operating procedures, performing frequent internal audits and assessments, and remaining current on regulatory changes. We also collaborate closely with regulatory agencies to ensure that our quality control methods and products comply with all applicable standards and recommendations.
We collaborate extensively with each client to comprehend their specific quality requirements and to create a quality control strategy that satisfies their objectives. This includes defining important quality traits, creating acceptance criteria, and implementing product-specific testing techniques and processes. We also give our clients frequent updates and progress reports to ensure that they are happy with our quality control systems.